Clinical Data
[ Consulting ]
We help Life Science extract value from data.
Future-ready Clinical Data Flow
Intilaris specializes in optimizing clinical data flow to help our clients to effectively collect, manage, understand, and interpret clinical data from both traditional and digital health sources.
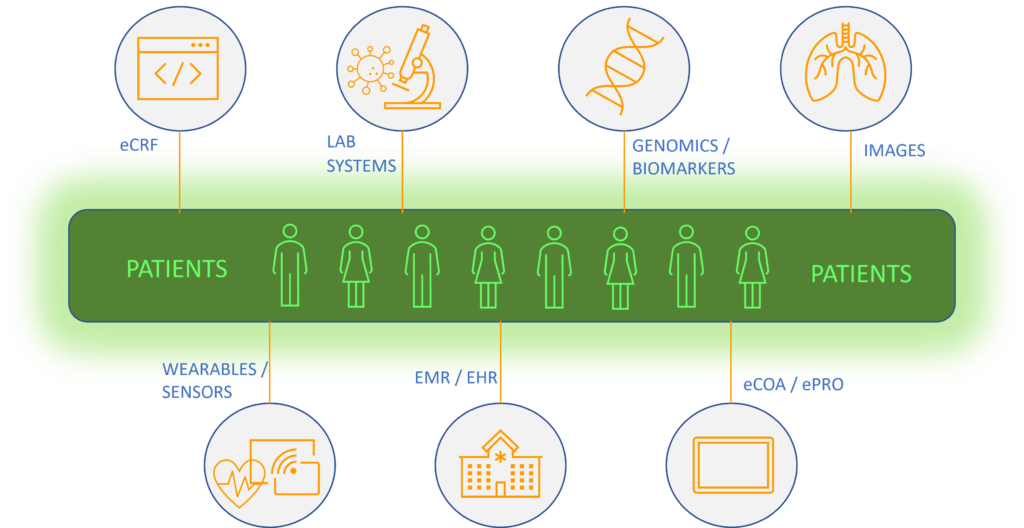
Data Domains
Clinical Study Protocol
Study Definitions Metadata
Data Collection
Centralized Study Monitoring
Medical Data Review
Safety Data Review
Medical Device Data
Study Data Tabulation Model
ADaM Datasets
Smart Query Management
Third Party Vendor Data
Medical Coding
Data Reconciliation
Data Management Reporting
Clinical Data Validation
Data Standards
CDISC Standards
Plan
PRM
Collect
CDASH
Organize
SDTM
Analyze
PRM
Data Exchange
SDM - XML
ODM - XML
Define - XML
Dataset - XML
CTR - XML
TransCelerate Digital Data Flow (DDF)
TransCelerate DDF vision is to optimize study processes through automation of manual and repetitive processes, connecting systems to drive improved study design, data quality, and reduce cycle times.
intilaris resources have been involved in the TransCelerate DDF initiative from the start of the initiative itself, but intilaris subject matter experts have contributed to the original ideas of Structuring Study Definition (SSD) and integrating it into downstream processes for accelerated implementation. We support our clients in introducing and implementing TransCelerate DDF into their clinical development organization.
Study Definition Repository
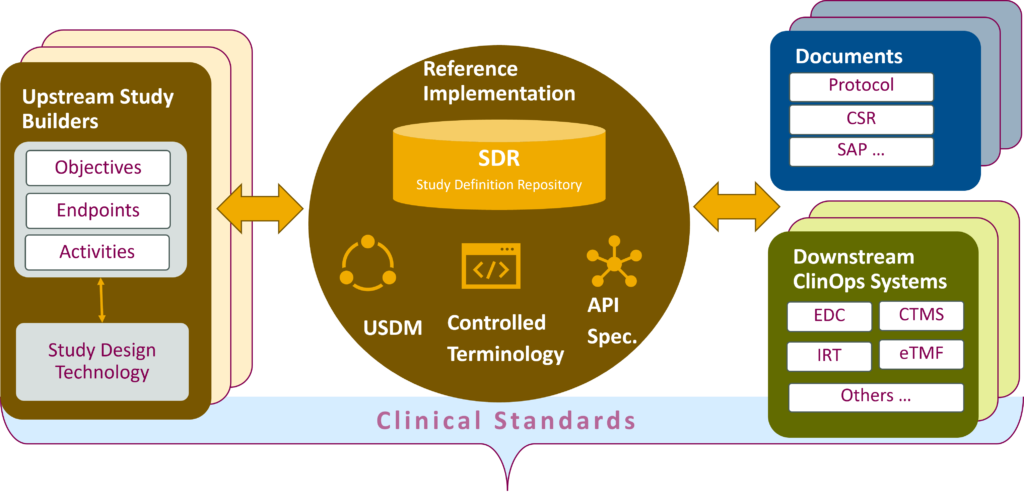
Unified Study Definition Model
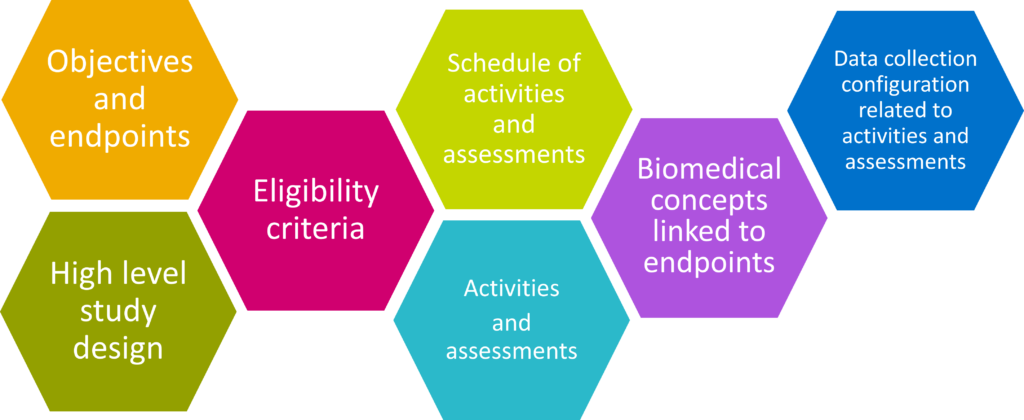
Clinical Data Products
Our clinical data subject-matter experts can help you identifying the best data-product opportunities demands in the clinical domain by merging the product-and-business perspective with the tech-and-data perspective.
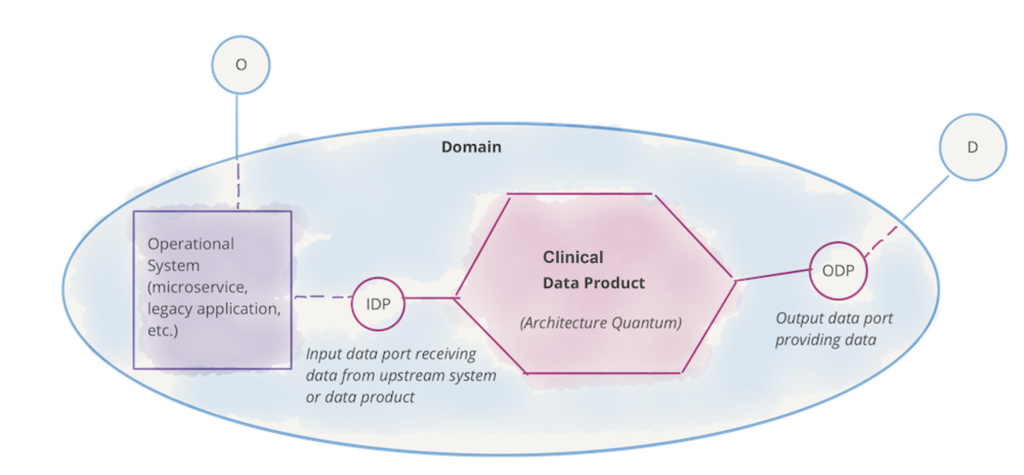
Data Integrity
Data integrity verifies the degree to which data collected at the point of capture, including the metadata, is complete, consistent and accurate throughout the data lifecycle. Integrity of source data is essential for meeting patient expectations and regulatory requirements.
We support you to ensure compliance with the regulatory guidance for data integrity. In other words, we ensure that data flow within customers IT systems maintains it’s integrity through managing the risks to impacted IT systems and the controls in place to mitigate those risks. We support you to manage the risks, controls and best practices for all types of IT systems:
- Equipment and instrumentation
- Enterprise-wide IT systems
- Local IT systems
Our approach to data integrity is supporting ALCOA principle, i.e. created data is attributable, legible, contemporaneous, original and accurate. Furthermore, the data is maintained in complete, consistent, enduring and available state throughout its lifecycle.

