Digital Clinical Study Protocol
[ Consulting ]
Leading experience with all technologies used in Clinical Development.
Digital clinical study protocol is a digital twin of the traditional clinical study protocol usually developed in clinical development organizations in a document form.
Traditional Clinical Study Protocol Development
Every clinical study investigation starts with the development of a clinical protocol. Typically, it is developed in a document form and details the conduct of the clinical trial. It specifies trial objectives, design, methodology, statistical approach, patient population, and the way the trial is organized. Clinical protocol ensures trial subjects’ safety and integrity of the collected data. According to the ICH Good Clinical Practice guidelines, a protocol should include the following topics:
- Title Page (General Information)
- Background Information
- Objectives/Purpose
- Study Design
- Selection and Exclusion of Subjects
- Treatment of Subjects
- Assessment of Efficacy
- Assessment of Safety
- Adverse Events
- Discontinuation of the Study
- Statistics
- Quality Control and Assurance
- Ethics
- Data handling and Record-keeping
- Publication Policy
- Project Timetable/Flowchart
- References
- Supplements/Appendices
Clinical protocol is a regulated document and as such is required for the conduct of the clinical trial. However, large number of researching pharma organizations are using the protocol document as a driver for most of the internal clinical operations processes. This is a bad idea, because in such a form, protocol document is very inefficient to transfer the right content to the given downstream process, requiring long information processing and extraction time as well as information interpretation. The interpretation of the clinical protocol document is particularly dangerous as it may undesirably influence the clinical trial conduct and consequently the trial outcome. Therefore, it is essential that we abstract our clinical development operation processes from the protocol document representation and employ digital clinical study protocol to drive all our downstream processes.
Digital Clinical Study Protocol
Digital clinical study protocol is a digital representation of the executable protocol information and as such it represents the single source of truth for the entire clinical development organization. When clinical study protocol is made available digitally to all downstream processes different process optimizations and innovations become available, like digital data flow, automated study build, site feasibility, target patient evaluations, etc.
Digital protocol becomes a true digital asset in the development organization, as it enables us to capture the knowledge about trial design, patients, amendments, etc. right in the context where it is relevant. This knowledge can be very beneficial to the organization in reducing the costs an accelerating the development timelines. For example, avoiding a single protocol amendment in study phase 3 (due to feasibility problems for instance) results in average saving of $500K in unbudgeted costs and adds an estimated 3 months to the unplanned development time [1].
Transform clinical development process for the digital age
To truly transform the clinical development process for the age of digital trials, we need to implement two core assets:
- Digital clinical study protocol model
- Lean and integrated development process
Digital clinical study protocol model instantiates an executable information container that holds all information required to conduct the clinical trial, for all relevant downstream processes.
The lean and integrated development process starts with an initial digital protocol and transforms and enriches its information content through development milestones. At each milestone, a set of digital protocol elements reaches its stability level and can be exchanged with downstream processes to front-load their activities. Such a lean clinical operations process provides the operational organization with agility to address the productivity and success rates of their clinical trials once they go into execution. Properly aligned with downstream processes and applying agile methods the organizations can instantly share the digital twin of their clinical protocol across the silos and provide end-to-end transparency in the entire value chain and all stakeholders.
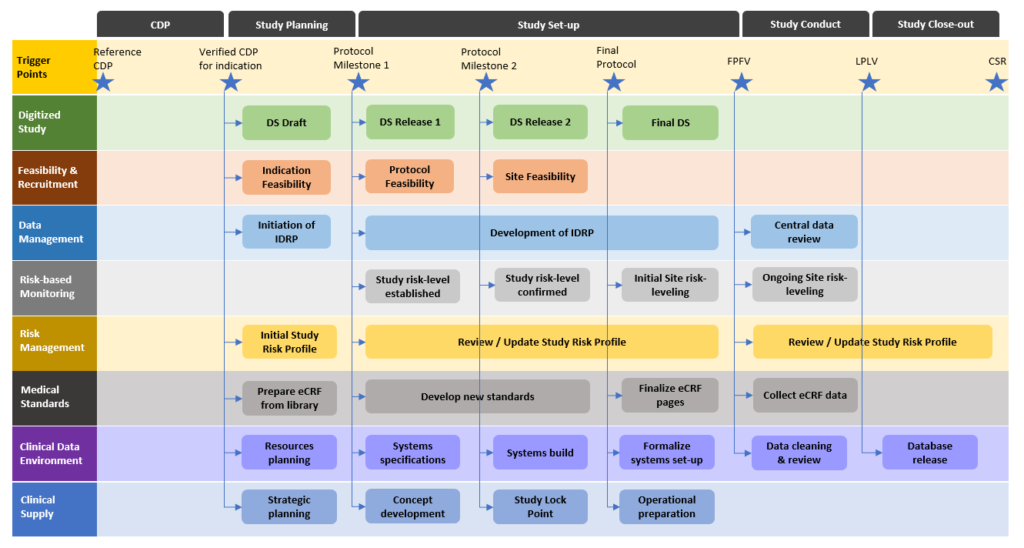
Intilaris has spent the last decade in working with large research organizations to transform the clinical development planning and design, including the lean and integrated protocol development. We have pioneered early ideas of electronic protocol representation and successfully implemented structured study design principles, and subsequently digitalized study planning, design and protocol development in large pharma organization.
Supporting you
Our support team is ready to help you in your digital transformation of the clinical operation processes using digital clinical study protocol.
For an in-depth conversation on this topic, please reach out to us.
intilaris LifeSciences – LinkedIn
[1] Getz, K. A., Stergiopoulos, S., Short, M., Surgeon, L., Krauss, R., Pretorius, S., Desmond, J., & Dunn, D. (2016). The Impact of Protocol Amendments on Clinical Trial Performance and Cost. Therapeutic innovation & regulatory science, 50(4), 436–441.

